Stool Test for Ova and Parasites (O&P)
A stool ova and parasites (O&P) exam is a critical laboratory test used to detect the presence of parasites or their eggs (ova) in a stool sample. This test is essential for diagnosing intestinal infections caused by these tiny organisms, which can inhabit and reproduce within the digestive system, leading to various gastrointestinal symptoms.
Intestinal parasites, including common types such as Giardia, Cryptosporidium, and Entamoeba histolytica (responsible for amebiasis), are often found in environments with poor sanitary conditions. These parasites can infect humans through contaminated water, soil, food, and surfaces. Notably, amebiasis is prevalent in tropical regions with inadequate sanitation, although parasites can also spread in developed countries, including the United States, through contaminated soil, undercooked meat, and untreated water sources like lakes, rivers, and swimming pools.
The stool sample test for ova and parasites (O&P) involves examining a stool sample under a microscope to identify the presence of parasites and their eggs or cysts, which are protective shells some parasites form at specific life stages. This test can reveal the cause of symptoms such as diarrhea, gas, and stomach cramps, which are indicative of parasitic infections. Infections can spread when individuals do not wash their hands properly after using the bathroom, leading to the contamination of surfaces and subsequent ingestion of the parasites through hand-to-mouth contact.
People with weakened immune systems, including infants, older adults, and those with conditions such as HIV or cancer, are particularly vulnerable to severe and prolonged parasitic infections. While healthy individuals may recover without treatment, those with compromised immunity may require medical intervention to eradicate the parasites and prevent serious health complications.
Collecting a stool sample for the O&P test typically involves parents or caregivers following specific instructions provided by healthcare professionals. The test results, which can take between 24 hours to several days, help doctors diagnose and treat gastrointestinal issues effectively, ensuring timely and appropriate care for those affected by parasitic infections.
Understanding and preventing parasitic infections through proper hygiene, safe food practices, and avoiding contaminated water sources are crucial steps in maintaining gastrointestinal health and preventing the spread of these infections.
Waste products excreted from the digestive tract consist primarily of water (up to 75%), along with indigestible residues, undigested food, partially digested but unabsorbed food, bile, epithelial cells, secretions from the digestive tract, inorganic materials, and bacteria. In adults, the normal daily fecal output ranges between 100 to 200 grams. Analyzing feces is instrumental in diagnosing gastrointestinal diseases.
Collection of Stool Sample for the Examination of Ova and Parasites
A random stool specimen, measuring at least 4 ml or 4 cm³, should be collected in a clean, dry container with a tightly fitting lid, such as a tin box, plastic container, glass jar, or waxed cardboard box. The sample must be promptly transported to the laboratory because trophozoites of Entamoeba histolytica rapidly degrade and alter their morphology. The recommended quantity is about 20-40 grams of formed stool or 5-6 tablespoons of watery stool. It is crucial to avoid contamination of the stool sample with urine, water, soil, or menstrual blood, as urine and water can destroy trophozoites, and soil can introduce extraneous organisms, complicating the examination process.
For optimal detection of parasites, it is essential to examine warm, freshly passed stools, ideally within one hour of collection. If an immediate examination is not possible, refrigerating the sample can help preserve its integrity. Alternatively, a fixative such as 10% formalin (for preserving eggs, larvae, and cysts) or polyvinyl alcohol (for preserving trophozoites and cysts, as well as for permanent staining) can be used when the specimen needs to be transported to a distant laboratory.
| Parameter | Amebic dysentery | Bacillary dysentery | |
|---|---|---|---|
| Cause | Entamoeba histolytica | Shigella (most common) | |
| Onset | Gradual | Acute | |
| Fever/vomiting | Not significant | Significant | |
| Appearance of fecal sample | Unformed with blood and mucus | Unformed with blood, mucus, and pus | |
| Microscopic examination of stool | |||
| Red cells | Clumps | Discrete | |
| Pus cells | Nil or few | Numerous | |
| Macrophages | Not seen | Many, some with ingested red cells | |
| Charcot-Leyden crystals | May be present | Not seen | |
| Trophozoites of E. histolytica | Present | Not seen | |
| Bacteria | Many, motile | Few, nonmotile | |
| Antigen test for E. histolytica | Positive | Negative | |
| Stool culture | Negative | Positive for Shigella | |
Protozoa
Entamoeba histolytica
Entamoeba histolytica is a protozoan parasite with a global distribution, particularly prevalent in tropical and subtropical regions. Transmission occurs via the fecal-oral route through ingestion of food or water contaminated with E. histolytica cysts.
Infection by E. histolytica can be asymptomatic or result in amebic dysentery or liver abscesses. The trophozoites invade the mucosa of the large intestine, proliferate in the submucosa, and create lateral spread, forming characteristic flask-shaped ulcers. Clinical manifestations include low-grade fever, diarrhea with blood and mucus, weight loss, and cramping abdominal pain, predominantly affecting the cecum, ascending colon, and rectosigmoid regions. Excessive granulation tissue at lesion sites may lead to intestinal constriction (ameboma), potentially mistaken for neoplasms.
In certain instances, the amebae penetrate portal vessels, reaching the liver to form abscesses. These abscesses can also develop in the lungs or brain.
Life Cycle of E. histolytica
Infection is acquired by ingesting food or water contaminated with infective quadrinucleate cysts of E. histolytica. In the small intestine, the cyst wall dissolves, releasing trophozoites. These trophozoites actively invade the large intestinal mucosa, lodge in the submucosa, multiply, and induce colitis. Extraintestinal dissemination to the liver and other sites may occur.
Under adverse conditions, trophozoites encyst within the intestinal lumen, forming cysts that are excreted in feces. These cysts can survive in moist environments for weeks to months, facilitating further fecal-oral transmission and perpetuating the life cycle.
Laboratory Diagnosis Techniques for E. histolytica Detection
Identification of Trophozoites and Cysts in Stool Examination
The detection of Entamoeba histolytica trophozoites in fecal samples is essential for diagnosing amebic dysentery. To enhance diagnostic accuracy, it is recommended to examine at least three fresh stool specimens. Trophozoites of E. histolytica range in size from 15 to 40 μm in diameter. In saline wet mounts, these trophozoites exhibit directional motility facilitated by rapidly forming pseudopodia. The cytoplasm of the trophozoite is characterized by an outer clear ectoplasm and an inner finely granular endoplasm.
A definitive diagnostic feature of E. histolytica trophozoites is the presence of ingested red blood cells. The nucleus, visible in iodine preparations, displays fine granules of peripheral nuclear chromatin evenly distributed along the nuclear membrane, with a small centrally located karyosome (Figure 1). It is important to note that motility is lost in iodine-mounted preparations.
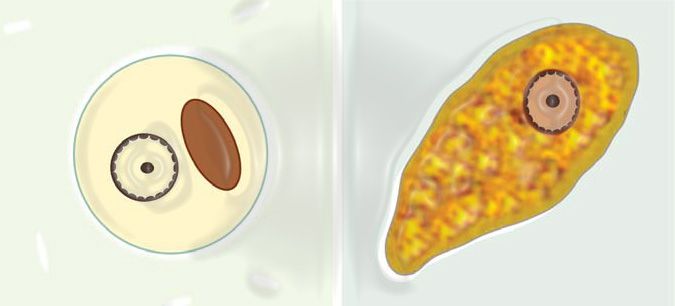
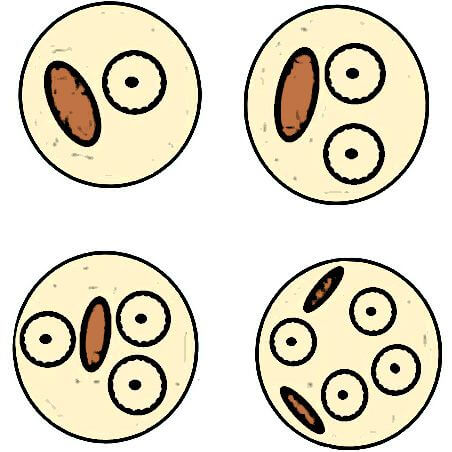
Cysts of Entamoeba histolytica are spherical and range from 10 to 15 μm in diameter. They can contain one to four nuclei, with mature cysts housing four. The nuclei exhibit a morphology similar to that of the trophozoite nucleus, characterized by a regular, thin nuclear membrane with finely granular peripheral chromatin. The karyosome is small and centrally located. Immature cysts may display chromatoid bodies, which are aggregates of ribosomes appearing as oblong structures with rounded ends, and glycogen clumps.
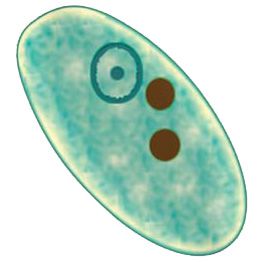
Entamoeba dispar is morphologically identical to Entamoeba histolytica but is non-pathogenic. The presence of red blood cells within trophozoites is indicative of E. histolytica. However, there are no morphological differences between the cysts of these two species. Therefore, when only cysts are observed in a stool examination, they are reported as “Cysts of E. histolytica/dispar”. Asymptomatic infections previously attributed to E. histolytica are now recognized to be caused by E. dispar. Differentiation between these organisms can be achieved using monoclonal antibodies.
Entamoeba histolytica can be definitively identified in a saline wet mount by observing clear directional motility and the presence of ingested red blood cells.
It is crucial to differentiate Entamoeba histolytica from other non-pathogenic amebae found in stool samples, such as Entamoeba coli, Entamoeba hartmanni, Endolimax nana, and Iodamoeba butschlii.
Wet mount examination of stool exhibits low sensitivity, ranging from 25% to 60%, and may yield false positives due to the presence of E. dispar. To accurately identify trophozoites, stool smears should be prepared and stained with trichrome stain.
Other Findings on Stool Examination
The presence of numerous red blood cells and very few white blood cells can aid in distinguishing amebic dysentery from bacillary dysentery. Additionally, Charcot-Leyden crystals may be observed. The differences between amebic and bacillary dysentery are detailed in Table 1.
Detection of Entamoeba histolytica Antigen in Stools
Direct detection of E. histolytica-specific antigens can be achieved using commercially available enzyme immunoassay tests. These tests are highly specific and sensitive (90%) and can differentiate E. histolytica from E. dispar. They are particularly useful when direct stool examination fails to identify organisms, yet clinical suspicion of intestinal amebiasis persists.
Detection of Entamoeba histolytica DNA
Polymerase chain reaction (PCR)-based assays enable the detection of E. histolytica-specific DNA.
Serologic Tests
Serologic tests are employed to detect antibodies against E. histolytica, supporting the diagnosis of invasive amebiasis (such as colitis, liver abscess, or ameboma) when organisms are not found in stool samples. Various serologic tests include latex agglutination, indirect hemagglutination, enzyme immunoassay, and counterimmunoelectrophoresis, with enzyme immunoassay being the preferred method due to its superior sensitivity and specificity. However, serologic tests can remain positive for many years post-infection, making it challenging to distinguish between recent and past infections.
Endoscopic Biopsy of Intestinal Ulcers
Endoscopic biopsy of intestinal ulcers can reveal E. histolytica trophozoites in approximately 50% of cases. Staining with periodic acid-Schiff (PAS) stain enhances the identification of the parasites.
Giardia intestinalis (lamblia)
Giardia intestinalis (also known as G. lamblia), a pathogenic intestinal protozoan, is found worldwide and is primarily transmitted via the fecal-oral route, often through contaminated water. Notably resistant to chlorine levels typically found in tap water, Giardia is commonly present in cold mountain streams. Giardiasis, the infection caused by Giardia, frequently affects individuals who camp or spend time outdoors, earning it the names “Backpacker’s diarrhea” and “beaver fever”. The infection can range from asymptomatic to causing mild diarrhea, and in severe cases, it can lead to significant symptoms such as persistent diarrhea, malabsorption, weight loss, and steatorrhea.
Life cycle of Giardia intestinalis (lamblia)
Giardia intestinalis undergoes two stages in its life cycle: the cyst and the trophozoite. Upon ingestion of cysts, the excystation process is triggered by gastric acid, releasing trophozoites. These trophozoites then migrate to the duodenum and proximal jejunum, where they attach to the mucosal lining and begin to replicate.
Laboratory Diagnosis Techniques for Giardia lamblia Detection
Demonstration of Trophozoites or Cysts
Giardia lamblia trophozoites are typically found in fresh, liquid stools, often within flakes of mucus, and they tend to appear in clusters. In contrast, cysts are more commonly observed in formed or loose stools.
If repeated fecal examinations fail to detect G. lamblia despite a strong clinical suspicion of giardiasis, duodenal aspirates may be collected. However, this invasive procedure is generally unnecessary for diagnosis.
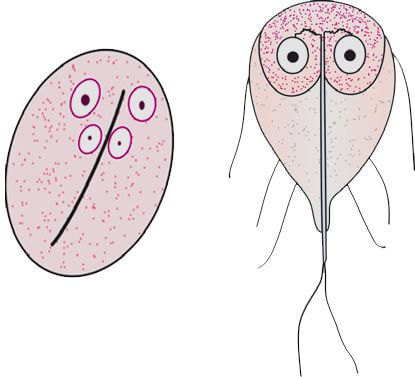
The trophozoites of G. lamblia are pear-shaped, measuring 12-15 μm in diameter, and possess four pairs of flagella, two large oval nuclei, two axonemes (axial filaments of flagella), and one or two curved median bodies. Their motility is described as resembling a “falling leaf”.
G. lamblia cysts are oval, measuring 8-12 μm in diameter, and contain four nuclei, axonemes, median bodies, and remnants of flagella (Figure 4).
Detection of G. lamblia Antigen in Stool Samples
The antigen of Giardia lamblia can be detected using enzyme immunoassay techniques, which exhibit high sensitivity (90-99%) and specificity (95-100%). This method is suitable as an initial diagnostic test for giardiasis, although stool examination remains essential for identifying other concurrent parasitic infections.
Direct Fluorescent Antibody Assay
The direct fluorescent antibody assay, commercially available in kit form, is both highly sensitive and specific. In this test, cysts are labeled with immunofluorescent antibodies and subsequently detected using a fluorescence microscope.
Coccidia
Coccidia includes Isospora belli, Cryptosporidium parvum, and Cyclospora cayetanensis, which are intestinal protozoans affecting humans. These organisms have a global distribution and are transmitted via the fecal-oral route through the ingestion of infective oocysts. They typically cause mild, self-limiting diarrheal illnesses; however, in immunocompromised individuals, such as those with acquired immunodeficiency syndrome (AIDS), they can lead to severe and prolonged diarrhea, potentially posing life-threatening risks.
Life cycle of Coccidia
Infection occurs when humans ingest infective oocysts. Once inside the host, sporozoites are released from the oocysts and infect the intestinal epithelial cells. Within these cells, the sporozoites multiply and form merozoites through asexual reproduction (fission or schizogony). These merozoites then infect other epithelial cells. Some merozoites differentiate into male and female gametes, which upon fertilization produce a zygote.
Encystation around the conjugating gametes results in the formation of oocysts. Sporozoites develop within these oocysts through sexual reproduction (sporogony). Finally, the oocysts are excreted in feces, contaminating food or water and continuing the transmission cycle.
Laboratory Diagnosis Techniques for Coccidia Detection
Examination of Stools for the Demonstration of Oocysts of I. belli, C. parvum, or C. cayetanensis
Isospora belli
Oocysts of Isospora belli can typically be identified in direct wet mounts of unstained fecal samples. Occasionally, the formalin-ether concentration technique is required. The oocysts are oval, measuring approximately 32 × 16 μm. Immature oocysts contain a granular zygote, while mature oocysts contain two sporocysts, each housing four sporozoites. When a fecal smear, prepared from the sediment after formalin-ether concentration, is stained with the modified Ziehl-Neelsen stain, oocysts stain uniformly red-pink. Additionally, under ultraviolet light, these oocysts exhibit autofluorescence.
Cryptosporidium parvum
Oocysts of Cryptosporidium parvum are challenging to detect in direct fecal wet mounts. They are more reliably demonstrated using the modified Ziehl-Neelsen staining technique on a concentrated fecal smear or through immunofluorescence methods. These oocysts are round to oval, measure 4-6 μm, and stain pink-red with the modified Ziehl-Neelsen stain.
Cyclospora cayetanensis
In direct wet mounts of feces, oocysts of Cyclospora cayetanensis measure 8-10 μm in diameter and exhibit a cluster of refractile globules, giving them a morula-like appearance. When stained with the modified Ziehl-Neelsen stain, they resemble C. parvum oocysts but are larger. Under ultraviolet light at 365 nm, these oocysts display intense blue autofluorescence.
Detection of Antigen in Stool Samples
Enzyme immunoassays are available for the detection of specific antigens of Cryptosporidium parvum. These assays offer greater sensitivity and specificity compared to traditional Ziehl-Neelsen staining methods.
Direct Fluorescent Antibody Assay
The direct fluorescent antibody assay, which is available for Cryptosporidium parvum, is highly sensitive and specific. In this assay, oocysts of Cryptosporidium are labeled with fluorescent antibodies and can be easily detected under a fluorescence microscope.
Microsporidia
Microsporidia are obligate intracellular protozoa that primarily cause opportunistic infections in immunocompromised patients, leading to persistent diarrhea and weight loss. The most common species infecting humans include Enterocytozoon bieneusi, Encephalitozoon intestinalis, and Encephalitozoon hellem.
Transmission and Pathogenesis
Infection occurs through the ingestion of spores. These organisms develop and proliferate within intestinal cells, forming infective spores. The rupture of host cells releases these spores, which can either infect new cells or be excreted in feces.
Clinical Manifestations
Beyond gastrointestinal involvement, some microsporidia species can cause keratoconjunctivitis, hepatitis, peritonitis, respiratory infections, and kidney disease. Due to their extremely small size, Microsporidia are not visible on wet mounts.
Laboratory Diagnosis Techniques for Microsporidia Detection
Modified Trichrome Stain of Stool Sample
The spores, which are extremely small (1-5 μm), stain red with the modified trichrome stain and may exhibit a characteristic transverse band.
Small Intestinal Biopsy for the Demonstration of Spores Within Intestinal Cells
A small intestinal biopsy can be performed to visualize the presence of spores within intestinal cells, confirming the infection.
Helminths
Ascaris lumbricoides (Roundworm)
Ascaris lumbricoides, commonly known as roundworm, represents the most prevalent helminthic infection affecting humans, with children being more susceptible than adults. The primary mode of transmission is through the fecal-oral route, typically from ingesting infective eggs. Within the human host, adult worms inhabit the small intestine, particularly the duodenum and jejunum.
Adult female worms are prolific egg layers, depositing approximately 200,000 eggs daily, which are then expelled through feces. These eggs exhibit remarkable durability, capable of remaining viable in soil for extended periods, often several years. Transmission occurs through various means, including the use of untreated human feces as fertilizer or through direct contamination from the hands of children at play. Adult worms can persist within the intestine of their human hosts for a duration of 1 to 2 years.
Life cycle of Ascaris lumbricoides (Roundworm)
Infection with Ascaris lumbricoides, or roundworm, occurs through ingestion of infective eggs found in contaminated food or transmitted via hands contaminated with fecal matter. Once ingested, these eggs hatch within the intestine, releasing larvae. These larvae penetrate the intestinal mucosa and enter the bloodstream, where they circulate and eventually reach the lungs.
Upon reaching the lungs, the larvae penetrate the alveolar walls and migrate up the respiratory tree. They are then coughed up and swallowed, subsequently returning to the small intestine. Within the small intestine, larvae mature into adult worms.
Adult female worms begin laying eggs, which are excreted in feces. These eggs require approximately 4 to 6 weeks in a suitable environment to become embryonated and infective. This life cycle perpetuates the transmission of Ascaris lumbricoides among human populations, especially in regions with poor sanitation practices.
Clinical Features
- Asymptomatic Infections: Infections with Ascaris lumbricoides may remain asymptomatic if the worm burden is light.
- Loeffler’s Syndrome: This syndrome occurs when larvae migrate through the lungs, causing symptoms such as cough, wheezing, eosinophilia (an increase in eosinophils in the blood), and irregular pulmonary densities visible on imaging.
- Local Effects: Ascaris lumbricoides can cause various local effects within the gastrointestinal tract. These include abdominal pain, diarrhea, and potentially serious complications such as intestinal obstruction due to a large mass of worms. In some cases, worms may penetrate the walls of the intestines, leading to intestinal perforation. Migration of worms into the pancreatic duct or common bile duct can cause obstruction, resulting in conditions like pancreatitis or obstructive jaundice. Furthermore, worms may travel from the bile duct to the liver, potentially causing abscess formation. There have also been cases where adult worms migrate to the appendix, triggering appendicitis.
- Malabsorption: Ascaris lumbricoides infections can interfere with the absorption of nutrients in the intestine, leading to malabsorption.
Laboratory Diagnosis Techniques for Ascaris lumbricoides Detection
Demonstration of Eggs of A. lumbricoides
Diagnosing an infection with Ascaris lumbricoides relies on identifying its characteristic eggs during stool examination. Eggs can typically be observed directly in a wet mount of feces in cases of moderate to heavy infections. For optimal detection, the recommended approach involves the formol-ethyl acetate sedimentation technique, which concentrates the eggs for clearer visualization.
In fecal samples, four distinct types of Ascaris lumbricoides eggs can be identified:
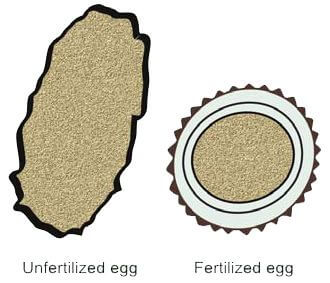
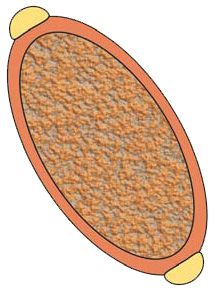
- Fertilized Eggs: These eggs are oval-shaped, measuring approximately 70 μ by 50 μ. They exhibit a characteristic appearance with an outer shell that is uneven, brown (stained by bile), and rough (mamillated). Inside, the egg contains a single central granular mass, known as the fertilized ovum.
- Unfertilized Eggs: Discharged by individual female worms, these eggs are slightly larger and more elongated than fertilized eggs, measuring about 90 μ in length. They feature a darker, more irregular outer shell and a thinner inner shell. The egg is filled with a mass of large refractile granules (Figure 5).
- Decorticated Eggs: These eggs lack the outer uneven shell, resembling eggs of hookworms in appearance.
Identification of Adult Worms
Adult worms of Ascaris lumbricoides may occasionally be passed spontaneously in feces and brought to the laboratory for identification. These adult worms are cylindrical or round, pinkish in color, and can measure about 15 cm for males and 30 cm for females in length. The diameter of these worms is approximately 0.5 cm. Notably, the tail of the male worm is curved, while that of the female is straight. Additionally, the anterior end (mouth) is characterized by three distinct lips.
Hookworms
The two primary species of hookworms affecting humans are Ancylostoma duodenale (the Old World hookworm) and Necator americanus (the New World hookworm).
Life Cycle of Hookworms
Infection with hookworms occurs when filariform larvae in the soil penetrate the skin, typically through the feet. Once inside, the larvae enter the bloodstream, are carried to the heart, and subsequently reach the lungs. From the lungs, they migrate up the respiratory tree and are swallowed. The larvae mature into adult worms in the small intestine, where they attach to the mucosa and feed on blood. Adult female worms lay eggs, which are excreted in feces. In the soil, these eggs release rhabditiform larvae that mature into infective filariform larvae.
Both Ancylostoma duodenale and Necator americanus infections are acquired through the penetration of the skin by infective filariform larvae. Additionally, Ancylostoma duodenale infections can also occur through the ingestion of infective larvae.
Clinical Features
Hookworm infections can present with the following clinical features:
- Ground Itch: This refers to the inflammation and intense itching at the site of larval penetration in the skin.
- Loeffler’s Syndrome: This condition arises due to the migration of larvae through the lungs.
- Iron Deficiency Anemia: Chronic blood loss from hookworm infection often leads to iron deficiency anemia. Adult worms attach to the small intestinal mucosa using teeth-like structures or cutting plates and consume blood. They frequently change their attachment sites (every 4-8 hours), causing continuous blood loss from the previous sites. On average, one adult Ancylostoma duodenale worm causes a loss of 0.15 ml of blood per day, whereas one Necator americanus worm causes a loss of 0.03 ml per day.
- Abdominal Pain and Diarrhea: These symptoms can occur when there is a high worm burden in the intestines.
Laboratory Diagnosis Techniques for Hookworm Detection
Demonstration of Hookworm Eggs
Diagnosis of hookworm infection primarily involves identifying hookworm eggs in stool samples. The preferred method is the formol-ethyl acetate sedimentation technique. In cases where this method is unavailable, a direct wet mount of a fecal sample can be used to detect eggs, particularly in moderate to heavy infections.
The eggs of Ancylostoma duodenale and Necator americanus are morphologically similar, measuring 50-75 μm in length and approximately 40 μm in width. These eggs are oval, colorless, and have a thin shell. Fresh stool samples typically reveal eggs containing 4-8 gray, granular cells.
If the stool sample is more than 12 hours old, the eggs will contain a folded rhabditiform larva, referred to as embryonated eggs. In samples older than 24 hours, free rhabditiform larvae will be present. It is crucial to differentiate these larvae from those of Strongyloides stercoralis, as hookworm larvae have a longer buccal cavity.

Other Laboratory Features
Blood tests frequently reveal eosinophilia in individuals with hookworm infections. Chronic blood loss due to the infection often leads to the development of microcytic hypochromic anemia. Additionally, tests for occult blood in the stool are typically positive, indicating ongoing intestinal bleeding.
Trichuris trichiura
Infection with Trichuris trichiura is contracted through the ingestion of infective eggs. Once ingested, the eggs hatch into larvae that develop into adult worms, which then attach to the mucosal lining of the intestines. The eggs produced by these adult worms are excreted in the feces and, under suitable environmental conditions, mature in the soil to become infective. Severe infections can lead to symptoms such as diarrhea with blood and mucus, iron deficiency anemia, and in some cases, rectal prolapse.
Diagnosis is made by identifying the characteristic eggs in stool samples. These eggs are typically 50 × 25 μ in size, yellow-brown, and barrel-shaped with a rounded, transparent plug at both poles (Figure 7). The eggs contain a central, uniformly granular mass. Quantification of the eggs is often performed to evaluate the severity of the infection.
Strongyloides stercoralis
Life Cycle of Strongyloides stercoralis
The life cycle of Strongyloides stercoralis is notably more intricate compared to other nematodes. Infection begins when infective filariform larvae in the soil penetrate the skin. These larvae enter the bloodstream, migrate to the lungs, penetrate the alveolar spaces, ascend the trachea, and are subsequently swallowed. Adult worms mature in the small intestine, where parthenogenetic female worms produce eggs. These eggs hatch in the small intestine, releasing rhabditiform larvae that are excreted with feces.
Occasionally, rhabditiform larvae mature into filariform larvae within the host, which can then penetrate the intestinal mucosa or perianal skin, entering the circulation—a process known as autoinfection. In the external environment, rhabditiform larvae can develop into infective filariform larvae capable of penetrating the skin and perpetuating the infection. If these larvae do not find a host, they mature into adult male and female worms in the soil, mate, and produce eggs. These eggs release rhabditiform larvae, which can then mature into infective filariform larvae, completing the cycle.
Clinical Features
- Dermatitis: Redness and itching at the site of filariform larva penetration.
- Loeffler’s Syndrome: Occurs due to the migration of larvae through the lungs, presenting with cough, wheezing, and pulmonary infiltrates.
- Acute Infection: Heavy infestation can lead to abdominal pain, diarrhea, and malabsorption.
- Chronic Infection: Persistent cases result in ongoing abdominal pain, diarrhea, and urticaria.
- Hyperinfection Syndrome: In immunocompromised individuals, severe hyperinfection due to autoinfection can develop, leading to pneumonia, neurologic complications, abdominal pain, septicemia, and shock, which can be fatal.
Laboratory Diagnosis Techniques for Strongyloides stercoralis Detection
Identification of Larvae of S. stercoralis
The diagnosis of Strongyloides stercoralis infection primarily involves the detection of rhabditiform larvae in fresh stool samples. Eggs of S. stercoralis are rarely observed in stool due to hatching and release of rhabditiform larvae in the intestine. These larvae, which measure 200-300 μm in length and 15 μm in width, are highly motile and characterized by two esophageal swellings, a prominent genital primordium, and a short buccal cavity. In older fecal samples, rhabditiform larvae of hookworms, which have a longer buccal cavity, might be mistaken for S. stercoralis larvae.
Larvae excretion is often inconsistent and the quantity may be minimal, leading to potential false negatives in stool examinations. Thus, concentration techniques such as formol-ethyl acetate sedimentation are recommended for more reliable results.
Additionally, duodenal fluid aspiration or the Entero-test (String test) can be employed for larval detection. The Entero-test involves swallowing a gelatin capsule containing a textured string, with one end taped to the cheek. The string reaches the duodenum, and after several hours, the capsule is retrieved. The bile-stained end of the string is wiped onto a glass slide for microscopic examination for larvae. In cases of disseminated infection, larvae may also be found in sputum samples.
For instances where larvae are not detected through fecal examination, duodenal aspirate, or the Entero-test, an enzyme immunoassay test that identifies IgG antibodies to S. stercoralis can be utilized. This serological test is valuable for confirming the presence of infection, although it cannot distinguish between current and past infections.
Enterobius vermicularis
Enterobius vermicularis, commonly known as pinworm, seatworm, or oxyurid, is a parasitic worm that infects humans globally, with a high prevalence among children. The primary mode of transmission is through the ingestion of food or water contaminated with infective eggs, typically via contaminated fingers.
Life Cycle of E. vermicularis
Upon ingestion, E. vermicularis eggs hatch in the intestine, releasing larvae that mature into adult worms in the cecum or appendix. At night, female worms migrate to the perianal area to deposit up to 15,000 eggs, causing intense itching. Scratching the irritated area leads to contamination of the fingers, which can result in self-infection when the eggs are transferred to the mouth. Infection can also occur through contact with contaminated clothing, bedding, and other objects.
Clinical Features
The hallmark symptom of enterobiasis is intense nocturnal perineal or perianal itching. Additionally, the presence of the worm in the appendix can sometimes lead to acute appendicitis. In females, the worm may invade the genital tract, causing vulvovaginitis and potentially leading to the formation of pelvic and peritoneal granulomas.
Special Technique for Collection and Demonstration of Pinworm Eggs
Due to the nocturnal migration of female pinworms to the perianal skin for egg deposition, pinworm eggs are typically found in the perianal skin folds rather than in routine stool samples. Two primary methods are used for egg collection:
- Transparent Adhesive Tape Test (Cellophane Tape Test): A piece of transparent adhesive tape is pressed onto the perianal skin to collect eggs. The tape is then placed sticky side down on a glass slide and examined under a microscope.
- Anal Swab Method: A cotton swab is rubbed around the anus, then rinsed in saline. The fluid is pipetted onto a glass slide and observed under a microscope with reduced illumination.
Specimens should be collected late at night or early in the morning before the patient has urinated, defecated, or bathed.
Laboratory Diagnosis Techniques for E. vermicularis (Human Pinworm) Detection
Diagnosis relies on the detection of eggs in samples collected from the perianal skin using the transparent adhesive tape method or by identifying adult worms. E. vermicularis eggs are oval, flattened on one side, measure 60 μm by 30 μm, and are colorless and transparent with a double-lined smooth shell. They contain a small granular mass (Figure 8) or a larva. Adult pinworms can be recovered from perianal skin folds using adhesive tape or may be found in children's feces. Adult worms are small, white, and motile, with males measuring approximately 0.5 cm and females about 1 cm in length.
Taenia solium
Taenia solium is prevalent in regions such as India, Pakistan, China, South Africa, and Latin America. The parasite is transmitted through the ingestion of raw or undercooked pork that contains infective cysticercus larvae, also known as cysticercus cellulose.
Life Cycle of Taenia solium
Infection occurs when humans, the definitive hosts, consume raw or undercooked pork containing encysted cysticercus larvae. The cysticercus scolex is released and attaches to the intestinal wall using its suckers and hooks. The cysticercus then matures into an adult tapeworm by adding multiple segments, known as proglottids, to its scolex. Adult tapeworms can reach lengths of 2 to 7 meters. Each proglottid acts as a hermaphroditic reproductive unit, producing numerous eggs. These egg-filled segments detach from the worm and are excreted in feces, contaminating the soil. Pigs, as intermediate hosts, ingest these eggs while feeding. The released embryos penetrate the pig's intestinal wall, enter the bloodstream, and develop into infective cysticercus larvae in the muscles.
Humans can also become intermediate hosts by ingesting food contaminated with eggs or through self-contamination (e.g., via contaminated fingers). In such cases, the embryos enter the bloodstream and develop into cysticerci at various body sites.
Clinical Features
- Intestinal Infection: of flat worm segments in their feces.
- Cysticercosis: This condition arises when humans accidentally ingest T. solium eggs or through autoinfection. Cysticerci develop in skeletal muscles, subcutaneous tissues, the heart, liver, brain, and other organs. The cysticerci, also known as bladder worms, are small cysts (less than 1.5 cm) containing clear fluid and an inverted scolex.
- Neurocysticercosis: This severe form involves the brain, causing seizures, increased intracranial pressure (due to cerebrospinal fluid obstruction by intraventricular cysts), psychiatric disturbances, and other neurological signs. Neurocysticercosis is a common cause of epilepsy in children in endemic areas and can sometimes lead to sudden death.
Laboratory Diagnosis Techniques for Taenia solium Detection
Examination of Feces for Taenia solium and Taenia saginata
- Identification of Eggs: Eggs of Taenia solium and Taenia saginata share identical morphology, making their differentiation challenging based on egg appearance alone. Each egg measures 30-40 μ in diameter, displaying a round to oval shape with a thick, brown wall marked by transverse lines. Within the egg, an embryo resides as a round granular mass containing three pairs of hooklets, surrounded by a delicate membrane (Figure 9). Occasionally, the egg is enclosed in a clear sac. Due to intermittent egg discharge by the tapeworm, detection may require repeated stool examinations and the use of the formol-ether concentration technique for reliable demonstration.
- Identification of Gravid Segments or Proglottids: Gravid segments or proglottids serve as crucial identifiers for species differentiation. A gravid segment, typically 13 mm × 8 mm in size, appears translucent and pale blue when flattened between two glass slides for examination. It features a central uterine stem with 8-13 lateral branches. In Taenia saginata, the uterine branches exceed 13, providing a distinctive characteristic for species identification.
- Identification of Scolex (Head): The scolex, or head, of a tapeworm is minute, resembling the size of a pinhead and rarely observed. Detailed examination under magnification reveals that the scolex of Taenia solium exhibits four suckers and a crown adorned with hooklets, distinguishing it from other species.
Diagnosis of Cysticercosis
Cysticercosis, a condition caused by the presence of cysticerci in tissues, can be diagnosed through several diagnostic modalities including serologic tests, radiologic studies, and biopsy of lesions.
Serologic tests such as the indirect hemagglutination assay demonstrate a sensitivity of approximately 80%. A titer equal to or greater than 1:64 is indicative of cysticercosis. For enhanced sensitivity and specificity, newer techniques like the glycoprotein immunoblot assay are employed, particularly effective in diagnosing neurocysticercosis.
Radiologic studies play a crucial role in diagnosis, with X-ray imaging facilitating the detection of calcified cysts. Computed tomography scans are especially valuable in identifying neurocysticercosis, providing detailed images of cystic lesions within the brain.
Taenia saginata
Taenia saginata, commonly known as the beef tapeworm, is distributed globally, with high prevalence observed in regions such as the Middle East, Africa, Asia, and Latin America. Transmission typically occurs through the consumption of raw or undercooked beef containing infective cysticercus bovis larvae.
The life cycle of Taenia saginata resembles that of Taenia solium, with notable differences: the animal host is cattle, the eggs are non-infectious to humans, thus human cysticercosis does not result from ingesting eggs, and segments of Taenia saginata also migrate to the perianal skin to deposit eggs.
Clinical Features
Clinical manifestations of Taenia saginata infection are generally mild. Symptoms may occasionally include abdominal pain and diarrhea, though these are typically not severe.
Laboratory Diagnosis Techniques for Taenia saginata (Beef Tapeworm) Detection
- Identification of Eggs: The eggs of Taenia saginata can be detected in fecal matter and perianal skin samples. Morphologically, these eggs are indistinguishable from those of Taenia solium.
- Identification of Gravid Segments: The gravid segments of T. saginata are 20 mm by 6 mm in size and ivory white in color. They possess a central uterine stem with 15 to 20 lateral branches, distinguishing them from T. solium.
- Identification of the Scolex (Head): The scolex of T. saginata is approximately 2 mm wide, featuring four suckers but lacking hooklets. This characteristic helps differentiate it from other species.
For a comprehensive overview of diagnostic laboratory tests for parasitic infections of the intestine, refer to Table 2.
| Test | Use |
|---|---|
| Direct saline mount | Trophozoites, cysts, ova, and larvae |
| Direct iodine mount | Protozoal cysts |
| Faecal concentration | Recovery of protozoal cysts, helminth ova and larvae |
| Cellophane tape technique | Ova of Enterobius vermicularis |
| Trichrome stain of fecal smear | Protozoal cysts and trophozoites |
| AFB stain of fecal smear | Oocysts of Cryptosporidium, Cyclospora, and Isospora |
| Detection of antigen in random stool sample | E. histolytica, G. lamblia, Cryptosporidium |
| Molecular methods based on polymerase chain reaction on stool sample | E. histolytica, G. lamblia, Cryptosporidium, Microsporidia, Cyclospora |
| Serologic methods | E. histolytica, S. stercoralis, Cysticercus |
References
- American Gastroenterological Association. AGA technical review on the evaluation and management of chronic diarrhea. Gastroenterology 1999;116: 1464-86.
- American Gastroenterological Association Medical Position Statement: Guidelines for the evaluation and management of chronic diarrhoea. Gastroenterology 1999;116:1461-3.
- Haque R, Huston CD, Hughes M, Houpt E, Petri, WA Jr. Amebiasis. New Engl J Med 2003;348:1565:73.
- Kucik CJ, Martin GL, Sortor BV. Common intestinal parasites. Am Fam Physician 2004;69:1161-8.
- Comment
- Posted by Dayyal Dg.